2025-06-04
Joint study by Duchesnay and PeriPharm on women's health
BLAINVILLE, QC, May 29, 2025 - Duchesnay, member of Duchesnay Pharmaceutical Group (DPG), winner of the 2024 Life Sciences Innovation Award by ADRIQ, in collaboration with PeriPharm, is proud to announce the results of a landmark study exploring the accessibility of innovative women's health products in Canada. The research exposes systemic gaps and significant barriers that prevent Canadian women from accessing the innovative therapies they need.
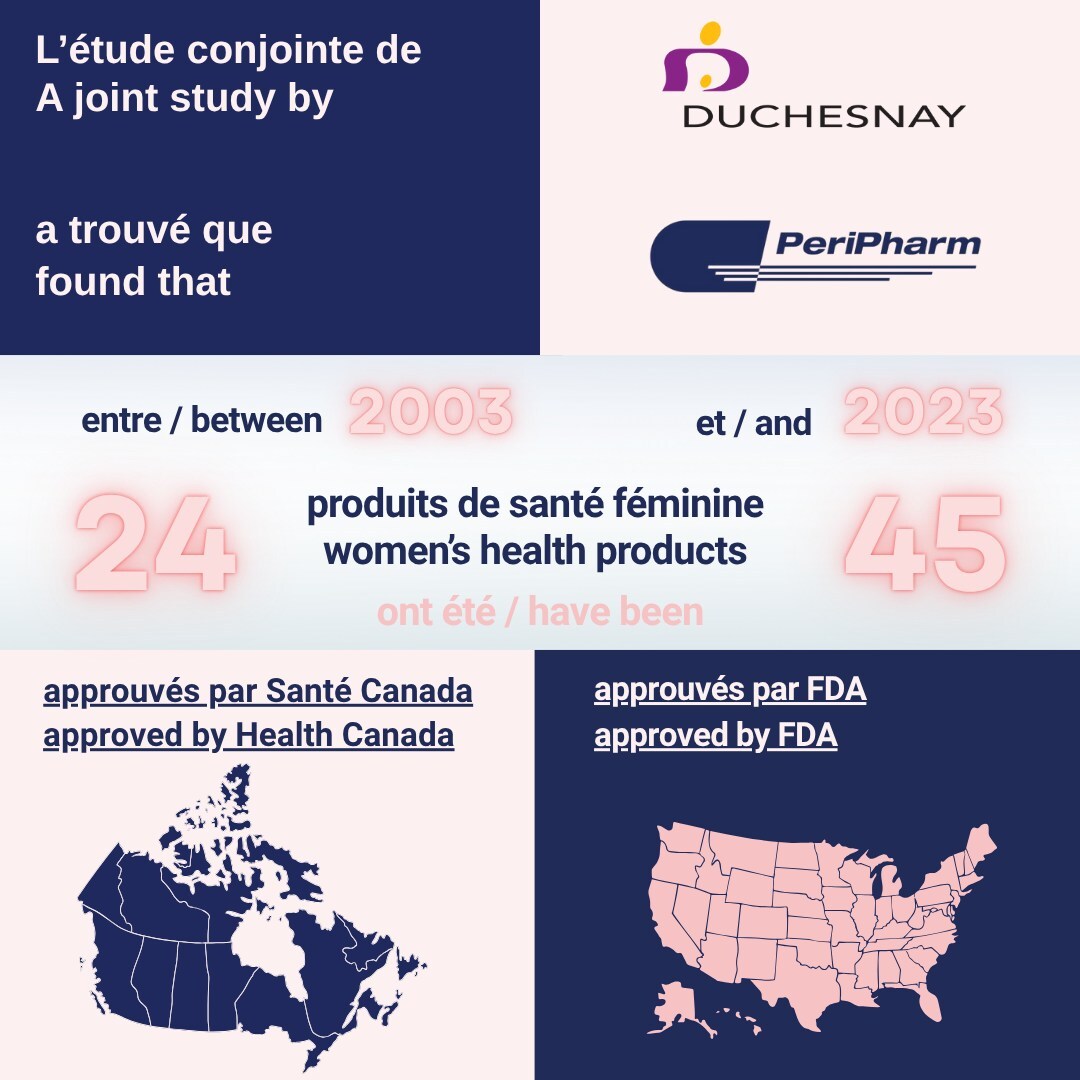
The study found that of the 45 women's health products approved by the U.S. Food and Drug Administration (FDA) from January 1, 2003, to December 31, 2023, only 24 (53%) had received regulatory approval by Health Canada as of July 2024 and only 13 of these products are currently reimbursed publicly.1
"These findings highlight a clear need to address inequities in how women's health is assessed, valued and prioritized," said Dany Hallé, Vice president, Commercial Affairs, DPG. "Striving to shape women's health ecosystem in Canada, we, at Duchesnay, believe that Canadian women deserve to have timely access to healthcare innovations."
Another important issue highlighted by researchers is the delays Canadian women face when it comes to approval and reimbursement processes for women's health therapies. Compared to the general access timeline for medications in Canada, the process for obtaining public coverage listing for women's health medications takes one year longer and can even exceed three years.
The study suggests that the current evidence-based Health Technology Assessment (HTA) framework may not fully capture the added value of innovative drugs in women's health, potentially contributing to delays or barriers in reimbursement and market access.
"This is a call to action," said Catherine Beauchemin, Ph.D., partner at PeriPharm. "This initiative marks an important first step toward improving access to innovations in women's health. To advance women's health outcomes in Canada, we must start by understanding their needs and preferences."
This study will serve as a benchmark and lay the groundwork for the development of a position paper co-written by leading experts in the domain addressing the challenges women ultimately face in the assessment of innovative medicines in Canada. This white paper would be hopefully available by year end.
1 In comparison, based on data between 2016 and 2020, 67% of all new active substance approval by FDA and/or EMA have been submitted to Health Canada.
ABOUT PERIPHARM
Founded in 2003, PeriPharm is a Canadian company specializing in pharmacoeconomics and outcomes research. The company's mission is to provide high-quality, diversified services to ensure optimal market access of health care innovations. PeriPharm's activity is built on the belief the best available therapies should be accessible to those who need it. As a leader in the field of health economics and data generation, PeriPharm has contributed to the success of several market access initiatives.
For more information about PeriPharm, please visit https://peripharm.com/en/ .
ABOUT DUCHESNAY
Duchesnay is a specialty pharmaceutical company with a long-standing commitment to women's health. Until recently, the company focused on filling the void in terms of scientific research and education and on developing pharmacological solutions that are safe and effective for use during pregnancy and breastfeeding.
Today, Duchesnay has broadened its portfolio of products to offer safe and effective therapeutic options that meet the health and quality of life needs of women and their family members at different stages of their lives. Believing that women around the world deserve to have access to specialized treatments for their conditions, Duchesnay now distributes its products internationally.
For more information about Duchesnay, please visit https://duchesnay.com/en/ .
The study found that of the 45 women's health products approved by the U.S. Food and Drug Administration (FDA) from January 1, 2003, to December 31, 2023, only 24 (53%) had received regulatory approval by Health Canada as of July 2024 and only 13 of these products are currently reimbursed publicly.1
"These findings highlight a clear need to address inequities in how women's health is assessed, valued and prioritized," said Dany Hallé, Vice president, Commercial Affairs, DPG. "Striving to shape women's health ecosystem in Canada, we, at Duchesnay, believe that Canadian women deserve to have timely access to healthcare innovations."
Another important issue highlighted by researchers is the delays Canadian women face when it comes to approval and reimbursement processes for women's health therapies. Compared to the general access timeline for medications in Canada, the process for obtaining public coverage listing for women's health medications takes one year longer and can even exceed three years.
The study suggests that the current evidence-based Health Technology Assessment (HTA) framework may not fully capture the added value of innovative drugs in women's health, potentially contributing to delays or barriers in reimbursement and market access.
"This is a call to action," said Catherine Beauchemin, Ph.D., partner at PeriPharm. "This initiative marks an important first step toward improving access to innovations in women's health. To advance women's health outcomes in Canada, we must start by understanding their needs and preferences."
This study will serve as a benchmark and lay the groundwork for the development of a position paper co-written by leading experts in the domain addressing the challenges women ultimately face in the assessment of innovative medicines in Canada. This white paper would be hopefully available by year end.
1 In comparison, based on data between 2016 and 2020, 67% of all new active substance approval by FDA and/or EMA have been submitted to Health Canada.
ABOUT PERIPHARM
Founded in 2003, PeriPharm is a Canadian company specializing in pharmacoeconomics and outcomes research. The company's mission is to provide high-quality, diversified services to ensure optimal market access of health care innovations. PeriPharm's activity is built on the belief the best available therapies should be accessible to those who need it. As a leader in the field of health economics and data generation, PeriPharm has contributed to the success of several market access initiatives.
For more information about PeriPharm, please visit https://peripharm.com/en/ .
ABOUT DUCHESNAY
Duchesnay is a specialty pharmaceutical company with a long-standing commitment to women's health. Until recently, the company focused on filling the void in terms of scientific research and education and on developing pharmacological solutions that are safe and effective for use during pregnancy and breastfeeding.
Today, Duchesnay has broadened its portfolio of products to offer safe and effective therapeutic options that meet the health and quality of life needs of women and their family members at different stages of their lives. Believing that women around the world deserve to have access to specialized treatments for their conditions, Duchesnay now distributes its products internationally.
For more information about Duchesnay, please visit https://duchesnay.com/en/ .